Why does Earth exist at all?
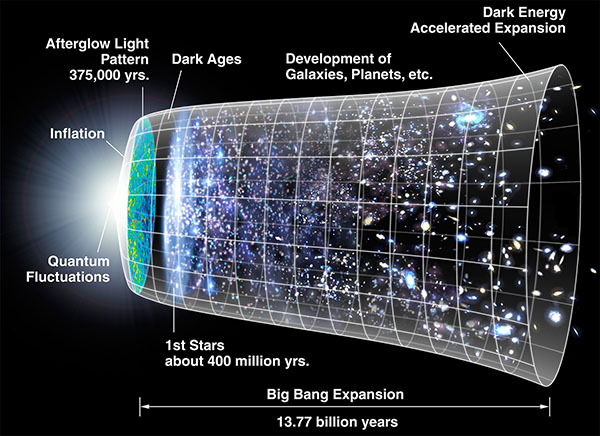
The best scientific model we have for understanding how the Earth exists begins with the Big Bang, the event that created space and time as we know and understand it, around 14 billion years ago. Scientists are interested in the questions of what possibly happened before the Big Bang and what caused the Big Bang to happen, but haven’t yet converged on any single best model for those. However, the Big Bang itself is well established by multiple independent lines of evidence and fairly uncontroversial.
The very early universe was a hot, dense place. Less than a second after the Big Bang, it was essentially a soup of primordial matter and energy. The energy density was so high that the equivalence of mass and energy (discovered by Albert Einstein) allowed energy to convert into particle/antiparticle pairs and vice versa. The earliest particles we know of were quarks, electrons, positrons, and neutrinos. The high energy density also pushed space apart, causing it to expand rapidly. As space expanded, the energy density reduced. The particles and antiparticles annihilated, converting back to energy, and this process left behind a relatively small residue of particles.
After about one millionth of a second, the quarks no longer had enough energy to stay separated, and bound together to form the protons and neutrons more familiar to us. The universe was now a plasma of charged particles, interacting strongly with the energy in the form of photons.
After a few minutes, the strong nuclear force could compete with the ambient energy level, and free neutrons bonded together with protons to form a few different types of atomic nuclei, in a process known as nucleosynthesis. A single proton and neutron could pair up to form a deuterium nucleus (an isotope of hydrogen, also known as hydrogen-2). More rarely, two protons and a neutron could combine to make a helium-3 nucleus. More rarely still, three protons and four neutrons occasionally joined to form a lithium-7 nucleus. Importantly, if two deuterium nuclei collided, they could stick together to form a helium-4 nucleus, the most common isotope of helium. The helium-4 nucleus (or alpha particle as it is also known in nuclear physics) is very stable, so the longer this process went on, the more helium nuclei were formed and the more depleted the supply of deuterium became. Ever since the Big Bang, natural processes have destroyed more of the deuterium, but created only insignificant additional amounts – which means that virtually all of the deuterium now in existence was created during the immediate aftermath of the Big Bang. This is important because measuring the abundance of deuterium in our universe now gives us valuable evidence on how long this phase of Big Bang nucleosynthesis lasted. Furthermore, measuring the relative abundances of helium-3 and lithium-7 also give us other constraints on the physics of the Big Bang. This is one method we have of knowing what the physical conditions during the very early universe must have been like.
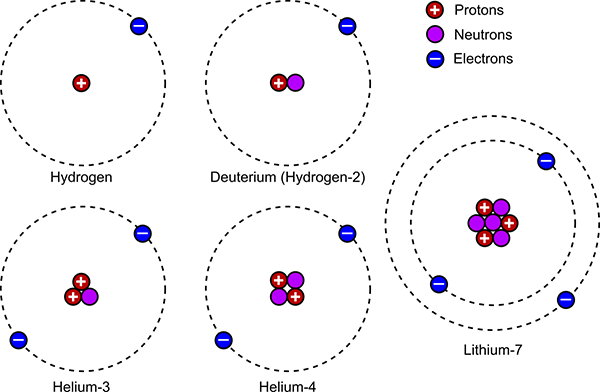
The numbers all point to this nucleosynthesis phase lasting approximately 380,000 years. All the neutrons had been bound into nuclei, but the vast majority of protons were left bare. At this time, something very important happened. The energy level had lowered enough for the electrostatic attraction of protons and electrons to form the first atoms. Prior to this, any atoms formed would quickly be ionised again by the surrounding energy. The bare protons attracted an electron each and become atoms of hydrogen. The deuterium nuclei also captured an electron to become atoms of deuterium. The helium-3 and helium-4 nuclei captured two electrons each, while the lithium nuclei attracted three. There were two other types of atoms almost certainly formed which I haven’t mentioned yet: hydrogen-3 (or tritium) and beryllium-7 – however both of these are radioactive and have short half-lives (12 years for tritium; 53 days for beryllium-7), so within a few hundred years there would be virtually none of either left. And that was it – the universe had its initial supply of atoms. There were no other elements yet.
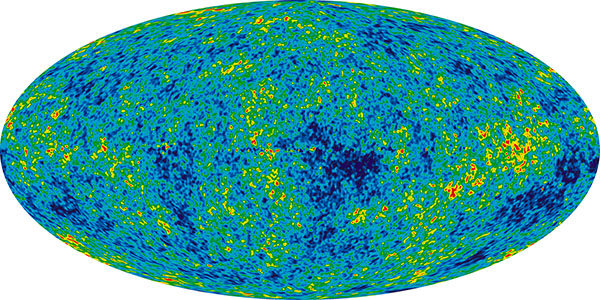
When the electrically charged electrons became attached to the charged nuclei, the electric charges cancelled out, and the universe changed from a charged plasma to an electrically neutral gas. This made a huge difference, because photons interact strongly with electrically charged particles, but much less so with neutral ones. Suddenly, the universe went from opaque to largely transparent, and light could propagate through space. When we look deep into space with our telescopes, we look back in time because of the finite speed of light (light arriving at Earth from a billion light years away left its source a billion years ago). This is the earliest possible time we can see. The temperature of the universe at this time was close to 3000 kelvins, and the radiation had a profile equal to that of a red-hot object at that temperature. Over the billions of years since, as space expanded, the radiation became stretched to longer wavelengths, until today it resembles the radiation seen from an object at temperature around 2.7 K. This is the cosmic microwave background radiation that we can observe in every direction in space – it is literally the glow of the Big Bang, and one of the strongest observational pieces of evidence that the Big Bang happened as described above.
The early universe was not uniform. The density of matter was a little higher in places, a little lower in other places. Gravity could now get to work. Where the matter was denser, gravity was higher, and these areas began attracting matter from the less dense regions. Over time, this formed larger and larger structures, the size of stars and planetary systems, galaxies, and clusters of galaxies. This part of the process is one where a lot of the details still need to be worked out – we know more about the earlier stages of the universe. At any rate, at some point clumps of gas roughly the size of planetary systems coalesced and the gas at the centre accreted under gravity until it became so massive that the pressure at the core initiated nuclear fusion. The clumps of gas became the first stars.
The first stars had no planets. There was nothing to make planets out of; the only elements in existence were hydrogen with a tiny bit of helium and lithium. But the nuclear fusion process that powered the stars created more elements: carbon, oxygen, nitrogen, silicon, sodium, all the way up to iron. After a few million years, the biggest stars had burnt through as much nuclear fuel in their cores as they could. Unable to sustain the nuclear reactions keeping them stable, they collapsed and exploded as supernovae, spraying the elements they produced back into the cosmos. The explosions also generated heavier elements: copper, gold, lead, uranium. All these things were created by the first stars.
The interstellar gas cloud was now enriched with heavy elements, but still by far mostly hydrogen. The stellar collapse process continued, but now as a star formed, there were heavy elements whirling in orbit around it. The conservation of angular momentum meant that elements spiralled slowly into the proto-star at the centre of the cloud, forming an accretion disc. Now slightly denser regions of the disc itself began attracting more matter due to their stronger gravity. Matter began piling up, and the heavier elements like carbon, silicon, and iron formed the first solid objects. Over a few million years, as the proto-star in the centre slowly absorbed more gas, the lumps of matter in orbit—now large enough to be called dust, or rocks—collided together and grew, becoming metres across, then kilometres, then hundreds of kilometres. At this size, gravity ensured the growing balls of rock were roughly spherical, due to hydrostatic equilibrium (previously discussed in a separate article). They attracted not only solid elements, but also gases like oxygen and hydrogen, which wrapped the growing protoplanets in atmospheres.
Eventually the star at the centre of this protoplanetary system ignited. The sudden burst of radiation pressure from the star blew away much of the remaining gas from the local neighbourhood, leaving behind only that which had been gravitationally bound to what were now planets. The closest planets had most of the gas blown away, but beyond a certain distance it was cold enough for much of the gas to remain. This is why the four innermost planets of our own solar system are small rocky worlds with thin or no atmospheres with virtually no hydrogen, while the four outermost planets are larger and have vast, dense atmospheres mainly of hydrogen and hydrogen compounds.
But the violence was not over yet. There were still a lot of chunks of orbiting rock and dust besides the planets. These continued to collide and reorganise, some becoming moons of the planets, others becoming independent asteroids circling the young sun. Collisions created craters on bigger worlds, and shattered some smaller ones to pieces.
The left over pieces of the creation of the solar system still collide with Earth to this day, producing meteors that can be seen in the night sky, and sometimes during daylight. (See also the previous article on meteor arrival rates.)
The process of planetary formation, all the way from the Big Bang, is relatively well understood, and our current theories are successful in explaining the features of our solar system and those we have observed around other stars. There are details to this story where we are still working out exactly how or when things happened, but the overall sequence is well established and fits with our observations of what solar systems are like. (There are several known extrasolar planetary systems with large gas giant planets close to their suns. This is a product of observational bias—our detection methods are most sensitive to massive planets close to their stars—and such planets can drift closer to their stars over time after formation.)
One major consequence of this sequence of events is that planets form as spherical objects (or almost-spherical ellipsoids). There is no known mechanism for the formation of a flat planet, and even if one did somehow form it would be unstable and collapse into a sphere.