There is a fairly straightforward experiment that we could do to establish that the Earth is a globe: Set off an enormous bomb.
Something of explosive power equivalent to 5-10 megatons of TNT. This is around 500 times as powerful as the Hiroshima atomic bomb. Ivy Mike, the first first full-scale thermonuclear hydrogen bomb detonation by the United States would serve nicely, with its yield of just over 10 megatons. The explosion of such a bomb would create a huge shockwave in the atmosphere, which would propagate outwards at the speed of sound in all directions.
This explosive shockwave would create a momentary barometric pressure spike as it passed by weather stations. Nearby stations would see a large pressure spike, while stations thousands of kilometres away would see a spike of the order of a millibar or two. This is strong enough that barometers all over the world should be able to detect the pressure wave passing through the atmosphere. You could then map the arrival time of the pressure wave, emanating in concentric circles from the bomb’s detonation point.
At the point on the Earth antipodal to where the bomb detonated, exactly on the opposite side of the globe, the pressure wave would converge from all directions approximately 16 hours later. This would demonstrate that there is an antipodes, a point on the opposite side of the spherical Earth. This phenomenon of the converging shockwave would not be observed on a flat Earth.
Unfortunately, this experiment is ethically dubious at best.
On 14 January, 2022, the volcano Hunga Tonga erupted explosively, approximately 65 km north of Tonga’s main island of Tongatapu. As I write this a week later, the scale of the destruction and damage to Tonga is still being assessed. According to various estimates, the explosion released somewhere in the range of 5-10 megatons of energy, making it the largest volcanic explosion of the 21st century to date. The eruption and the resulting tsunamis are of course a human tragedy, but they also provide opportunities for knowledge and understanding.
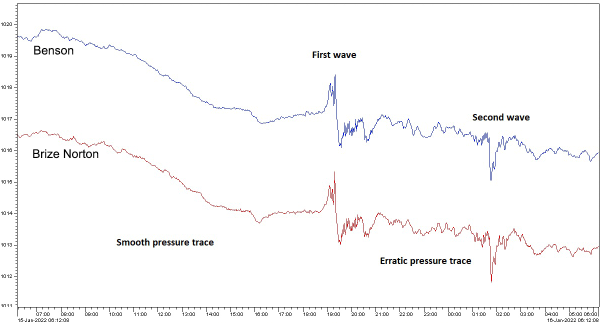
The explosion occurred at 17:15 local time on 14 January (04:15 UTC, 15 January). Soon after, meteorological stations across the world were recording changes in barometric pressure due to the shockwave. The Australian Bureau of Meteorology released this trace of mean sea level pressure changes caused by the eruption.

The figure shows the propagation time delays as the shockwave travels at the speed of sound. Norfolk Island is 1900 km from Tonga and the wave arrived after about 1 hour 50 minutes. This gives the average speed of sound to be around 330 m/s, which is within the expected range. Perth is 6840 km from Tonga and the wave arrived after a little more than 6 hours. This gives the average speed of sound to be around 310 m/s, which is also within the expected variation given pressure and humidity differences.
Australia is relatively close to Tonga, globally speaking. The UK Met Office also released traces of barometric pressure as recorded at a couple of weather stations near Oxford in the UK.
The wave arrived after about 14.5 hours. Given the Tonga-Oxford distance of 16,600 km, this gives a speed of 314 m/s, again well within the expected range. More interesting is the arrival of a second wave a little less than 7 hours later. If we use the same speed of sound, this gives a propagation distance of 24,300 km. This is more than half way around the world. In fact, within the expected range of variation of propagation speed, this is close enough to the distance from Tonga to Oxford, travelling in the opposite direction—the long way around the world.
And this is exactly what we are seeing here. The first recorded wave travelled the shortest path from Tonga to Oxford, while the second wave went in the opposite direction, the long way, and so took 7 hours longer to arrive.
This by itself is strong evidence of the spherical shape of the Earth. But even more dramatic are videos showing propagation of the atmospheric shock wave, as imaged by weather satellites. In the days after the eruption, several meteorologists and atmospheric scientists downloaded publicly available images from geostationary weather satellites. They took infrared images, which show cloud patterns even in night time, and subtracted pairs of images taken at intervals 15 minutes apart, to show the differences between the state of the atmosphere in that time frame.
One resulting video using a satellite over the Pacific Ocean shows the site of the explosion in Tonga and the shockwave rippling outwards in concentric circles on the globe. As the shockwave propagates, you can see it getting weaker.
A second video uses a satellite over Africa. This shows the shockwave converging on the opposite side of the world. The eruption occurred at latitude 20.550°S, longitude 175.385°W. The antipodal point on the opposite side of the world is thus latitude 20.550°N, longitude 4.615°E, in southern Algeria. And in this video, you can see the shockwave converging on that point in Algeria, and then expanding again, producing the second wave that was later recorded in Oxford. (This video is sped up relative to the first video, to make it easier to see the shockwave propagation, because it is significantly weaker and more difficult to see at the slower speed.)
This provides clear visual evidence that the shockwave expands from one point on the Earth’s surface and, after propagating in all directions at approximately the same speed, it converged back to a point on the opposite side of the globe, equidistant from the origin in all directions.
We may not be able to reproduce the experiment with a bomb in good conscience, but nature often provides fascinating ways for us to test our understanding of the world and the universe.